Evaluate two-sample tests
jhsiao999
2018-11-15
Last updated: 2018-11-16
workflowr checks: (Click a bullet for more information)-
✔ R Markdown file: up-to-date
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
-
✔ Environment: empty
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
-
✔ Seed:
set.seed(20181115)The command
set.seed(20181115)was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible. -
✔ Session information: recorded
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
-
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.✔ Repository version: c3707b4
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can usewflow_publishorwflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.Ignored files: Ignored: .DS_Store Ignored: .Rhistory Ignored: .Rprofile Ignored: .Rproj.user/ Ignored: .sos/ Ignored: analysis/.DS_Store Ignored: analysis/.sos/ Ignored: docs/figure/ Ignored: dsc/.DS_Store Ignored: dsc/.sos/ Ignored: dsc/benchmark/.DS_Store Ignored: dsc/modules/.DS_Store Unstaged changes: Modified: dsc/benchmark.query.R
Expand here to see past versions:
Overview
We applied two-sample tests for mean difference to simulated datasets.
The methods applied are:
- glm poisson
- glm quasipoisson
- t-test
- wilcoson tet
The simulated data were made from experimental datasets as described below.
Draw (sample without replacement) p genes and N samples from the experimental dataset.
For the
Correlated case, assign samples to Group 1 or Group 2.For the
Uncorrelated case, permute sample labels for each gene, then in the permuted sample, assign samples to Group 1 or Group 2.
In addition, the parameter setting is:
Sample size (n1 vs n2): 50 vs 50, 100 vs 100
Number of genes (p): 1,000
Regarding the experimental data:
single cell RNA-seq data from 10x genomics technology
The dataset used in the simulation include CD14+ Monocytes and CD8 T cells, a total of 787 samples and 946 genes.
The complete dataset include 2,700 single cells and 32,738 genes from 8 immune cell types: B cells, CD4 T cells, CD8 T cells, CD14+ Monocytes, Dendritic cells, FCGR3A+ Monocytes, Megakaryocytes, NK cells.
We filtered the original data to include samples that are detected in > 200 genes and genes that are detected in > 20% of cells.
Results
library(dscrutils)
#setwd("~/Dropbox/GitHub/dsc-log-fold-change/dsc")
dir_dsc <- "/Users/joycehsiao/Dropbox/GitHub/dsc-log-fold-change/dsc/benchmark"
out <- dscquery(dir_dsc,
c("get_data", "get_data.n1", "get_data.n2", "method", "method.p"))Loading dsc-query output from CSV file.
Reading DSC outputs:
- method.p: vectors not extracted (set max.extract.vector = 946 to extract)plotRest <- function(dir_dsc, dscoutput,
sim_case=c("random_sample", "random_gene"),
sample_size=c(50,100),
title_label, plot=T, return_res=T) {
#dscoutput <- out; sim_case="random_sample"; sample_size=50
out.sub <- dscoutput[dscoutput$get_data==sim_case & dscoutput$get_data.n1==sample_size,]
res <- vector("list",4)
for (i in 1:nrow(out.sub)) {
# print(i)
fl <- readRDS(file.path(dir_dsc,
paste0(as.character(out.sub$method.p[i]), ".rds")))
res[[i]] <- data.frame(method = as.character(out.sub$method)[i],
n1_n2 = paste0(out.sub$get_data.n1[i],".",
out.sub$get_data.n2[i]),
pval = fl$p,
stringsAsFactors = F)
}
names(res) <- as.character(out.sub$method)
if (plot) {
cols <- c("gray50", "forestgreen", "blue", "orange")
par(mfrow=c(2,2))
for (i in 1:length(res)) {
hist(res[[i]]$pval, main="",
xlab = "p-values", ylab = "Frequency",
nclass = 20, col=cols[i])
title(main=names(res)[i])
}
title(title_label, outer=T, line=-1)
qq <- lapply(1:length(res), function(i) {
qqplot(x=runif(sample_size*2,0,1), y=res[[i]]$pval, plot.it=F)
})
plot(qq[[1]]$x, qq[[1]]$y, col = "gray50", cex=.7, pch = 16,
xlab = "Uniform(0,1)", ylab = "Empirical distribution",
main = "QQ-plot")
points(qq[[2]]$x, qq[[2]]$y, col = "forestgreen", cex=.7, pch = 16)
points(qq[[2]]$x, qq[[2]]$y, col = "blue", cex=.7, pch = 16)
points(qq[[3]]$x, qq[[3]]$y, col = "orange", cex=.7, pch = 16)
abline(0,1, col = "black")
title(title_label, outer=TRUE, line=-1)
}
if (return_res) {
return(res)
}
}
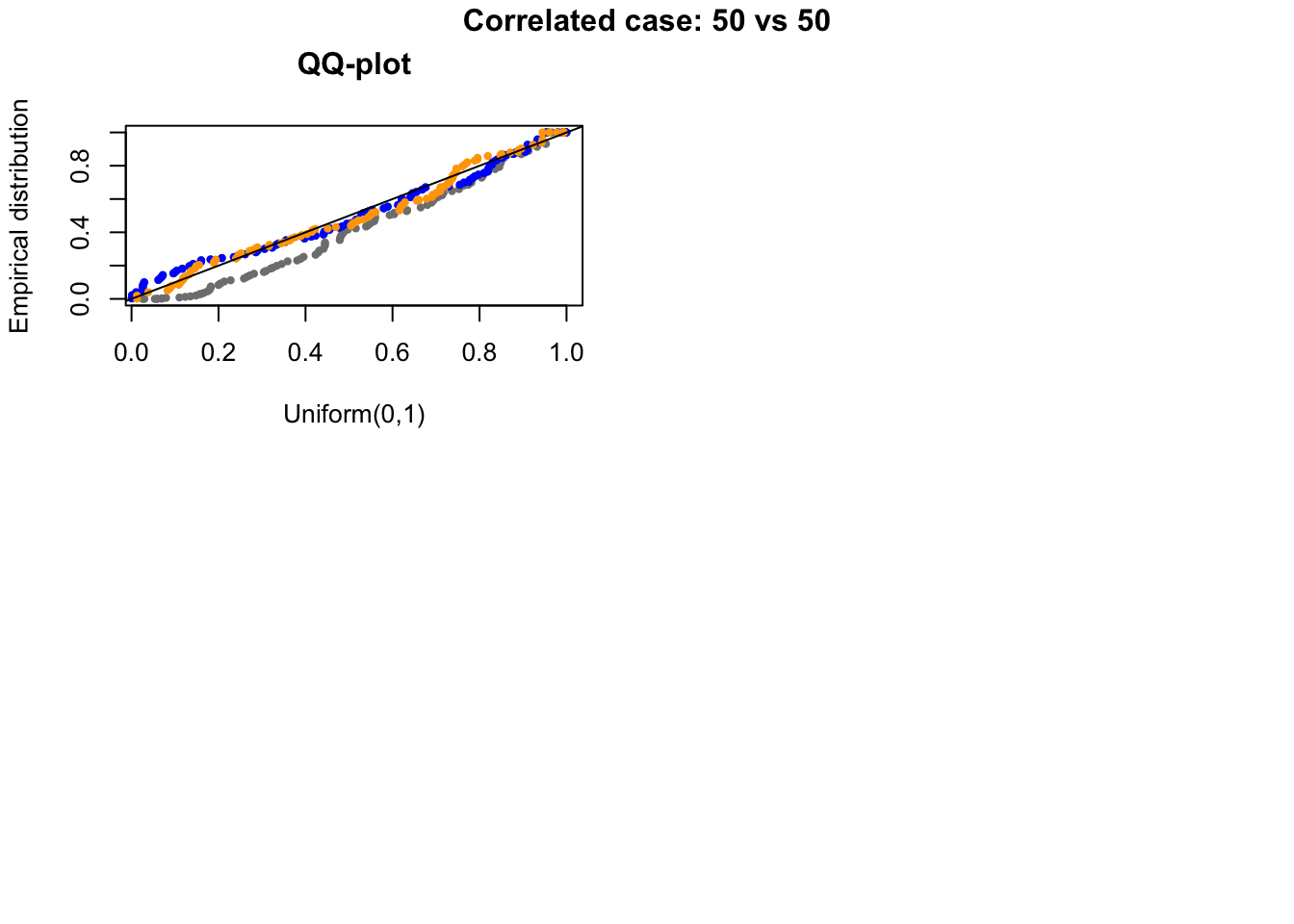
out.corr <- plotRest(dir_dsc=dir_dsc, dscoutput=out, sim_case="random_sample",
sample_size=50,
title_label="Correlated case: 50 vs 50", plot=T, return_res=T)
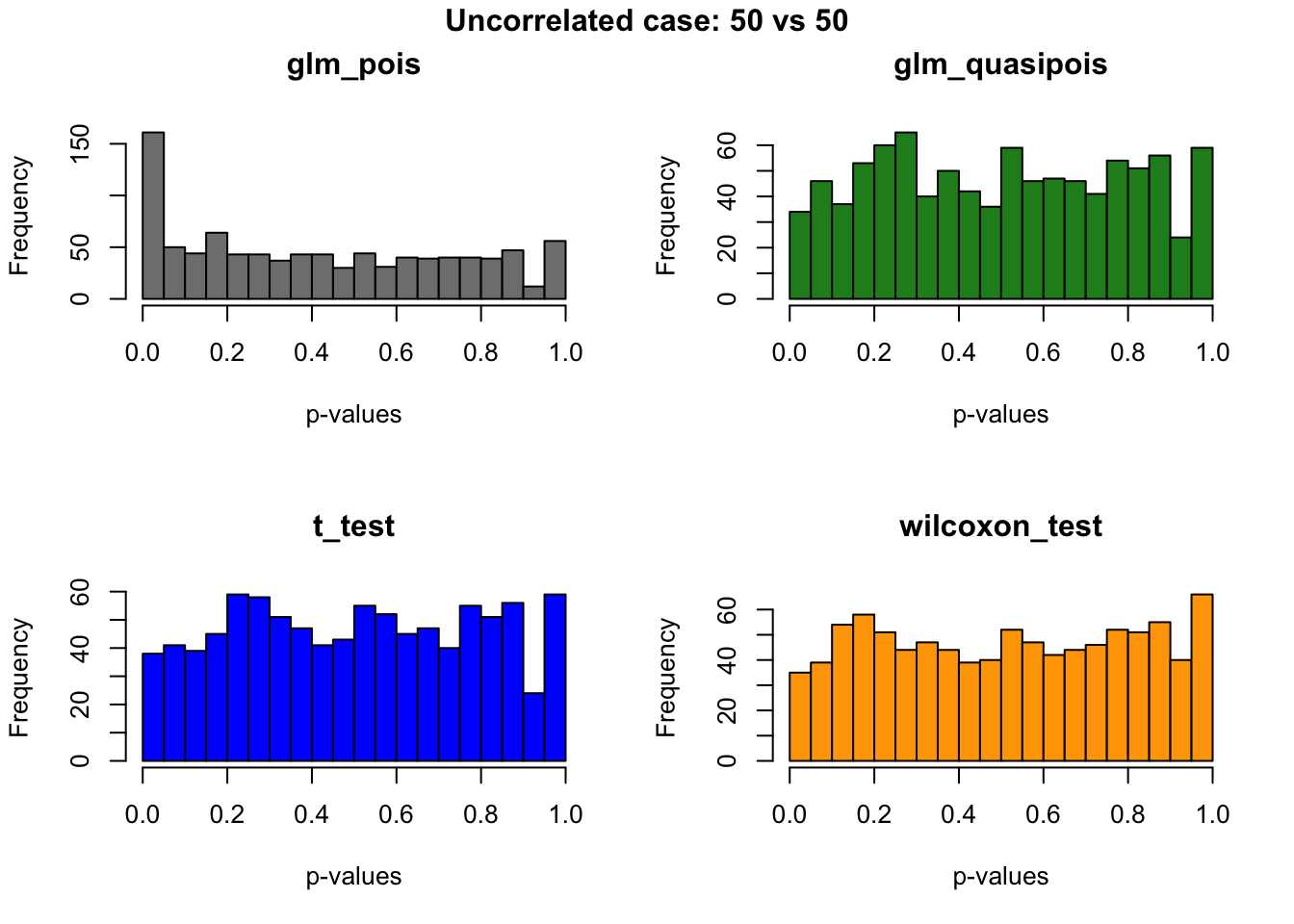
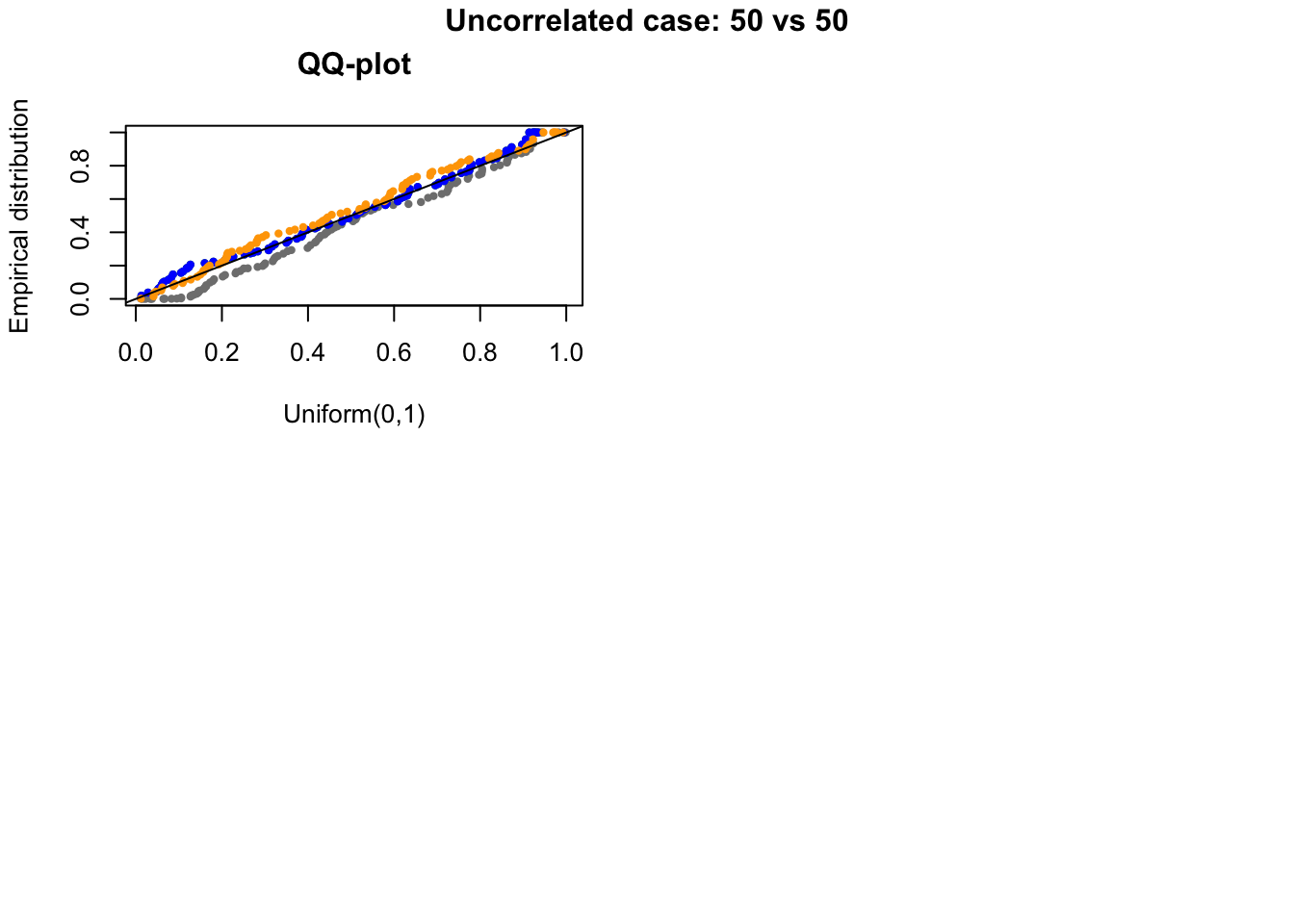
out.uncorr <- plotRest(dir_dsc=dir_dsc, dscoutput=out, sim_case="random_gene",
sample_size=50,
title_label="Uncorrelated case: 50 vs 50", plot=T, return_res=T)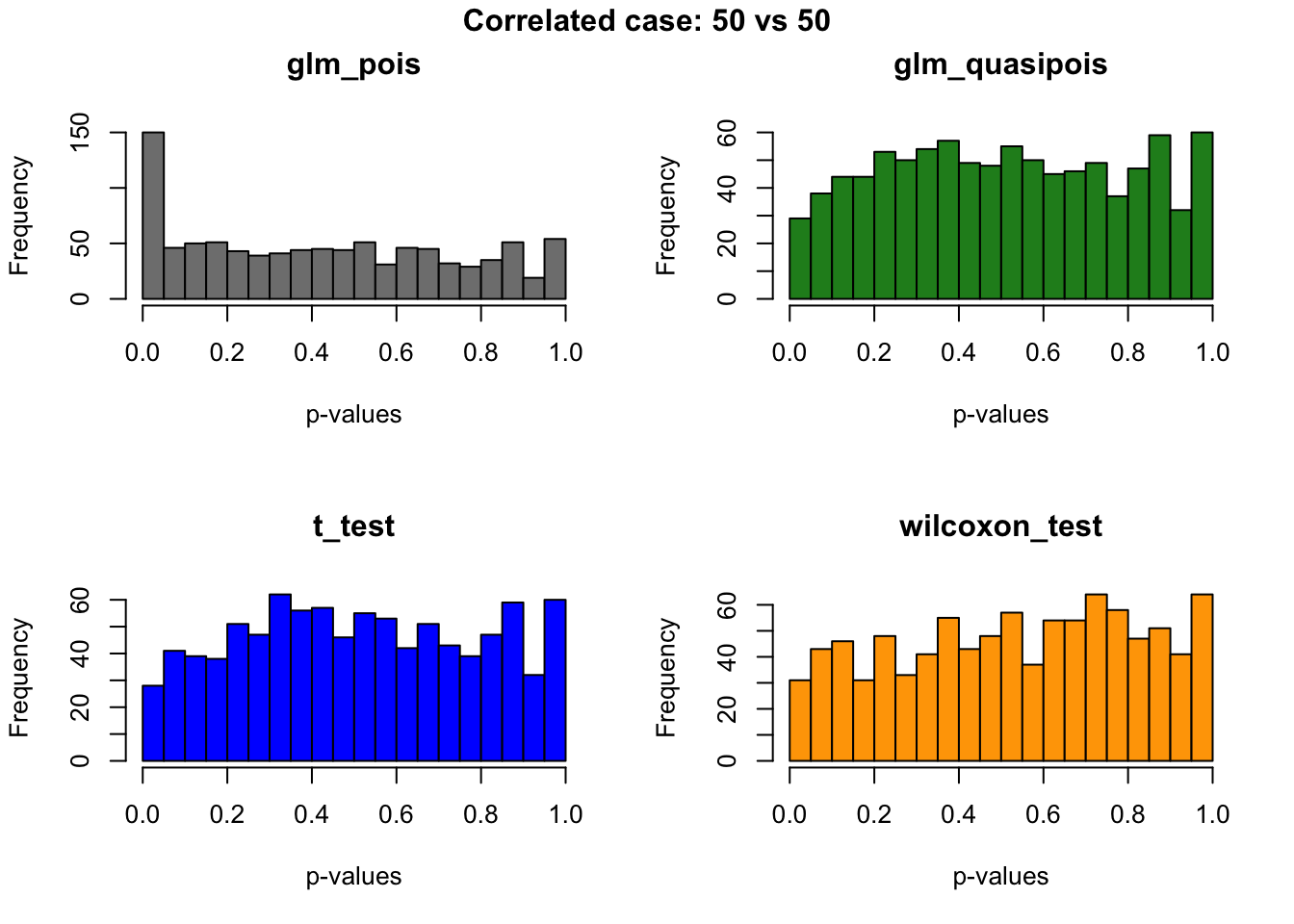
Session information
sessionInfo()R version 3.4.1 (2017-06-30)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS High Sierra 10.13
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.4/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.4/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] dscrutils_0.2.7.11
loaded via a namespace (and not attached):
[1] workflowr_1.1.1 Rcpp_1.0.0 digest_0.6.18
[4] rprojroot_1.3-2 R.methodsS3_1.7.1 backports_1.1.2
[7] magrittr_1.5 git2r_0.23.0 evaluate_0.12
[10] stringi_1.2.4 whisker_0.3-2 R.oo_1.22.0
[13] R.utils_2.7.0 rmarkdown_1.10 tools_3.4.1
[16] stringr_1.3.1 yaml_2.2.0 compiler_3.4.1
[19] htmltools_0.3.6 knitr_1.20 This reproducible R Markdown analysis was created with workflowr 1.1.1