Overlap Molecular QTLs
Briana Mittleman
9/6/2018
Last updated: 2018-09-06
workflowr checks: (Click a bullet for more information)-
✔ R Markdown file: up-to-date
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
-
✔ Environment: empty
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
-
✔ Seed:
set.seed(12345)The command
set.seed(12345)was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible. -
✔ Session information: recorded
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
-
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility. The version displayed above was the version of the Git repository at the time these results were generated.✔ Repository version: 57005d9
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can usewflow_publishorwflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.Ignored files: Ignored: .DS_Store Ignored: .Rhistory Ignored: .Rproj.user/ Ignored: output/.DS_Store Untracked files: Untracked: analysis/ncbiRefSeq_sm.sort.mRNA.bed Untracked: analysis/snake.config.notes.Rmd Untracked: data/18486.genecov.txt Untracked: data/APApeaksYL.total.inbrain.bed Untracked: data/RNAkalisto/ Untracked: data/Totalpeaks_filtered_clean.bed Untracked: data/YL-SP-18486-T-combined-genecov.txt Untracked: data/YL-SP-18486-T_S9_R1_001-genecov.txt Untracked: data/bedgraph_peaks/ Untracked: data/bin200.5.T.nuccov.bed Untracked: data/bin200.Anuccov.bed Untracked: data/bin200.nuccov.bed Untracked: data/clean_peaks/ Untracked: data/comb_map_stats.csv Untracked: data/comb_map_stats.xlsx Untracked: data/combined_reads_mapped_three_prime_seq.csv Untracked: data/gencov.test.csv Untracked: data/gencov.test.txt Untracked: data/gencov_zero.test.csv Untracked: data/gencov_zero.test.txt Untracked: data/gene_cov/ Untracked: data/joined Untracked: data/leafcutter/ Untracked: data/merged_combined_YL-SP-threeprimeseq.bg Untracked: data/nom_QTL/ Untracked: data/nom_QTL_opp/ Untracked: data/nuc6up/ Untracked: data/other_qtls/ Untracked: data/peakPerRefSeqGene/ Untracked: data/perm_QTL/ Untracked: data/perm_QTL_opp/ Untracked: data/reads_mapped_three_prime_seq.csv Untracked: data/smash.cov.results.bed Untracked: data/smash.cov.results.csv Untracked: data/smash.cov.results.txt Untracked: data/smash_testregion/ Untracked: data/ssFC200.cov.bed Untracked: data/temp.file1 Untracked: data/temp.file2 Untracked: data/temp.gencov.test.txt Untracked: data/temp.gencov_zero.test.txt Untracked: output/picard/ Untracked: output/plots/ Untracked: output/qual.fig2.pdf Unstaged changes: Modified: analysis/28ind.peak.explore.Rmd Modified: analysis/cleanupdtseq.internalpriming.Rmd Modified: analysis/dif.iso.usage.leafcutter.Rmd Modified: analysis/explore.filters.Rmd Modified: analysis/peak.cov.pipeline.Rmd Modified: analysis/peakOverlap_oppstrand.Rmd Modified: analysis/pheno.leaf.comb.Rmd Modified: analysis/test.max2.Rmd Modified: code/Snakefile
Expand here to see past versions:
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 57005d9 | Briana Mittleman | 2018-09-06 | make qqplot |
| html | 584548c | Briana Mittleman | 2018-09-06 | Build site. |
| Rmd | e2f5e81 | Briana Mittleman | 2018-09-06 | fix run code |
| html | f92a58f | Briana Mittleman | 2018-09-06 | Build site. |
| Rmd | 46b7343 | Briana Mittleman | 2018-09-06 | add overlap analysis with code to subset |
I will use this to overlap my QTLs with the other molecular QTLs already identified in the same individuals. First pass I will subset my nuclear and total nomial qtls by the snps with pvals less than .05 in each of the sets and make a qqplot.
Create reg QTL files
I want to create a python script that takes in which type of qtl and a pvalue and subsets the full file for snps that pass that filter.
subset_qtls.py
def main(inFile, outFile, qtl, cutoff):
fout=open(outFile, "w")
ifile=open(inFile, "r")
cutoff=float(cutoff)
qtl_types= ['4su_30', '4su_60', 'RNAseq', 'RNAseqGeuvadis', 'ribo', 'prot']
if qtl not in qtl_types:
raise NameError("QTL arg must be 4su_30, 4su_60, RNAseq, RNAseqGeuvadis, ribo, or prot")
elif qtl=="4su_30":
target=4
elif qtl=="4su_60":
target=5
elif qtl=="RNAseq":
target=6
elif qtl=="RNAseqGeuvadis":
target=7
elif qtl=="ribo":
target =8
elif qtl=="prot":
target=9
for num,ln in enumerate(ifile):
if num > 0 :
line_list = ln.split()
chrom=line_list[0][3:]
pos=line_list[1]
rsid=line_list[2]
geneID=line_list[3]
val = line_list[target].split(":")[0]
if val == "NA":
continue
else:
val = float(val)
if val <= cutoff:
fout.write("%s:%s\t%s\t%s\t%f\n"%(chrom, pos, rsid, geneID,val))
if __name__ == "__main__":
import sys
qtl = sys.argv[1]
cutoff= sys.argv[2]
inFile = "/project2/gilad/briana/threeprimeseq/data/otherQTL/summary_betas_ste_100kb.txt"
outFile = "/project2/gilad/briana/threeprimeseq/data/otherQTL/summary_betas_ste_100kb.%s%s.txt"%(qtl, cutoff)
main(inFile, outFile, qtl, cutoff)I can run this to subset by each qtl at .05
run_subsetQTLs05.sh
#!/bin/bash
#SBATCH --job-name=run_subsetqtl05
#SBATCH --account=pi-gilad
#SBATCH --time=24:00:00
#SBATCH --output=run_subsetqtl05.out
#SBATCH --error=run_subsetqtl05.err
#SBATCH --partition=gilad
#SBATCH --mem=12G
#SBATCH --mail-type=END
module load Anaconda3
source activate three-prime-env
#qtls=('4su_30', '4su_60', 'RNAseq', 'RNAseqGeuvadis', 'ribo', 'prot')
for i in 4su_30 4su_60 RNAseq RNAseqGeuvadis ribo prot; do
python subset_qtls.py $i .05
done
Load data
library(tidyverse)── Attaching packages ─────────────────────────────────────── tidyverse 1.2.1 ──✔ ggplot2 3.0.0 ✔ purrr 0.2.5
✔ tibble 1.4.2 ✔ dplyr 0.7.6
✔ tidyr 0.8.1 ✔ stringr 1.3.1
✔ readr 1.1.1 ✔ forcats 0.3.0── Conflicts ────────────────────────────────────────── tidyverse_conflicts() ──
✖ dplyr::filter() masks stats::filter()
✖ dplyr::lag() masks stats::lag()library(workflowr)This is workflowr version 1.1.1
Run ?workflowr for help getting startedlibrary(reshape2)
Attaching package: 'reshape2'The following object is masked from 'package:tidyr':
smithslibrary(readr)nuc.nom=read.table("../data/nom_QTL_opp/filtered_APApeaks_merged_allchrom_refseqGenes_pheno_Nuclear_NomRes_onetenth.txt", header = F, stringsAsFactors = F)
colnames(nuc.nom)= c("peakID", "snpID", "dist", "nuc_pval", "slope")
QTL_names=c("snpID", "snpid2","Gene", "pval")
fourSU30= read.table("../data/other_qtls/summary_betas_ste_100kb.4su_30.05.txt", header=F, stringsAsFactors = F, col.names = QTL_names)
fourSU60=read.table("../data/other_qtls/summary_betas_ste_100kb.4su_60.05.txt", header=F, stringsAsFactors = F, col.names = QTL_names)
RNAseq=read.table("../data/other_qtls/summary_betas_ste_100kb.RNAseq.05.txt", header=F, stringsAsFactors = F, col.names = QTL_names)
guevardis=read.table("../data/other_qtls/summary_betas_ste_100kb.RNAseqGeuvadis.05.txt", header=F, stringsAsFactors = F, col.names = QTL_names)
ribo=read.table("../data/other_qtls/summary_betas_ste_100kb.ribo.05.txt", header=F, stringsAsFactors = F, col.names = QTL_names)
prot=read.table("../data/other_qtls/summary_betas_ste_100kb.prot.05.txt", header=F, stringsAsFactors = F, col.names = QTL_names)Filter nuc by other QTLs
Overlap the files:
fourSU30AndNuc= fourSU30 %>% inner_join(nuc.nom, by="snpID") %>% select(snpID, nuc_pval)
fourSU30_unif=runif(nrow(fourSU30AndNuc))
fourSU60AndNuc= fourSU60 %>% inner_join(nuc.nom, by="snpID") %>% select(snpID, nuc_pval)
fourSU60_unif=runif(nrow(fourSU60AndNuc))
RNAAndNuc= RNAseq %>% inner_join(nuc.nom, by="snpID") %>% select(snpID, nuc_pval)
RNAseq_unif=runif(nrow(RNAAndNuc))
GuevAndNuc= guevardis %>% inner_join(nuc.nom, by="snpID") %>% select(snpID, nuc_pval)
guev_unif=runif(nrow(GuevAndNuc))
riboAndNuc= ribo %>% inner_join(nuc.nom, by="snpID") %>% select(snpID, nuc_pval)
ribo_unif=runif(nrow(riboAndNuc))
protAndNuc= prot %>% inner_join(nuc.nom, by="snpID") %>% select(snpID, nuc_pval)
prot_unif=runif(nrow(protAndNuc))Plot overlapping QTLs
Plot results:
qqplot(-log10(runif(nrow(nuc.nom))), -log10(nuc.nom$nuc_pval),ylab="-log10 Nuclear nominal pvalue", xlab="Uniform expectation", main="Nuclear Nominal pvalues for all snps")
points(sort(-log10(fourSU30_unif)), sort(-log10(fourSU30AndNuc$nuc_pval)), col="Red")
points(sort(-log10(fourSU60_unif)), sort(-log10(fourSU60AndNuc$nuc_pval)), col="Orange")
points(sort(-log10(RNAseq_unif)), sort(-log10(RNAAndNuc$nuc_pval)), col="Yellow")
points(sort(-log10(guev_unif)), sort(-log10(GuevAndNuc$nuc_pval)), col="Green")
points(sort(-log10(ribo_unif)), sort(-log10(riboAndNuc$nuc_pval)), col="Blue")
points(sort(-log10(prot_unif)), sort(-log10(protAndNuc$nuc_pval)), col="Purple")
abline(0,1)
legend("topleft", legend=c("All SNPs", "4su 30", "4su 60", "RNAseq", "Guevadis RNA", "Ribo", "Col"), col=c("black", "red", "orange", "yellow", "green", "blue", "purple"), pch=19)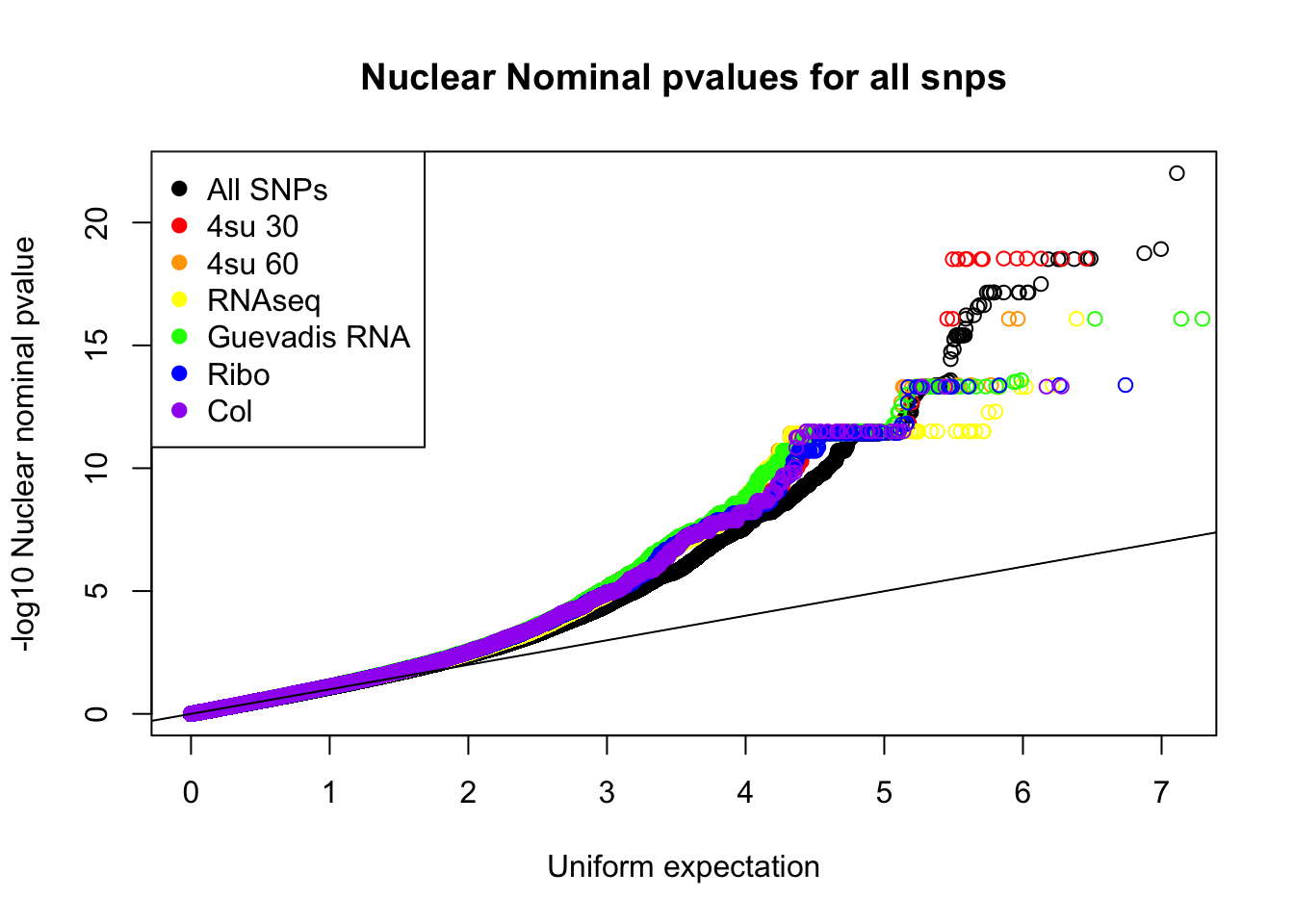
Session information
sessionInfo()R version 3.5.1 (2018-07-02)
Platform: x86_64-apple-darwin15.6.0 (64-bit)
Running under: macOS Sierra 10.12.6
Matrix products: default
BLAS: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRblas.0.dylib
LAPACK: /Library/Frameworks/R.framework/Versions/3.5/Resources/lib/libRlapack.dylib
locale:
[1] en_US.UTF-8/en_US.UTF-8/en_US.UTF-8/C/en_US.UTF-8/en_US.UTF-8
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] reshape2_1.4.3 workflowr_1.1.1 forcats_0.3.0 stringr_1.3.1
[5] dplyr_0.7.6 purrr_0.2.5 readr_1.1.1 tidyr_0.8.1
[9] tibble_1.4.2 ggplot2_3.0.0 tidyverse_1.2.1
loaded via a namespace (and not attached):
[1] tidyselect_0.2.4 haven_1.1.2 lattice_0.20-35
[4] colorspace_1.3-2 htmltools_0.3.6 yaml_2.2.0
[7] rlang_0.2.2 R.oo_1.22.0 pillar_1.3.0
[10] glue_1.3.0 withr_2.1.2 R.utils_2.7.0
[13] modelr_0.1.2 readxl_1.1.0 bindrcpp_0.2.2
[16] bindr_0.1.1 plyr_1.8.4 munsell_0.5.0
[19] gtable_0.2.0 cellranger_1.1.0 rvest_0.3.2
[22] R.methodsS3_1.7.1 evaluate_0.11 knitr_1.20
[25] broom_0.5.0 Rcpp_0.12.18 scales_1.0.0
[28] backports_1.1.2 jsonlite_1.5 hms_0.4.2
[31] digest_0.6.16 stringi_1.2.4 grid_3.5.1
[34] rprojroot_1.3-2 cli_1.0.0 tools_3.5.1
[37] magrittr_1.5 lazyeval_0.2.1 crayon_1.3.4
[40] whisker_0.3-2 pkgconfig_2.0.2 xml2_1.2.0
[43] lubridate_1.7.4 assertthat_0.2.0 rmarkdown_1.10
[46] httr_1.3.1 rstudioapi_0.7 R6_2.2.2
[49] nlme_3.1-137 git2r_0.23.0 compiler_3.5.1
This reproducible R Markdown analysis was created with workflowr 1.1.1